CSSD Infection Control Management: A 20-Year Director’s Cornerstone Guide
Process discipline, environmental control, monitoring, traceability—and how hospitals keep sterile supply safe.
CSSD is not a back-office service. It is a high-risk safety system. The expected outcome is uncompromising: clinical instruments must achieve 100% qualified reprocessing before use.
Table of Contents
- Overview: What CSSD Controls (and why it is core to IPC)
- CSSD Service Models & Centralized Governance
- Facility Layout: Zoning & One-Way Flow (Dirty → Clean → Sterile)
- Environmental Requirements: Pressure, ACH, Temperature, Humidity & Lighting
- The 10-Step Workflow & Risk Points
- Process Controls: Management Key Points by Step
- Quality Monitoring: Cleaning / Disinfection / Sterilization / B-D
- Traceability & Record Retention
- Continuous Improvement & Recall Governance
- External Instruments & Implants: Governance Requirements
- Common Failures Seen in Audits (Real-World Risks)
- Manager’s Readiness Checklist
- FAQ
- Recommended Internal & External References
1) Overview: What CSSD Controls (and why it is core to IPC)
The Central Sterile Supply Department (CSSD) is responsible for cleaning, disinfection, sterilization, and sterile supply of reusable medical devices across the hospital. Its quality directly determines patient safety and is a critical part of the infection-control chain.
- Zero-tolerance outcome Sterilization qualification must reach 100% before clinical use.
- Complex process chain Decontamination involves multiple tightly coupled steps.
- System role CSSD is often called the “liver of the hospital” due to its risk-filtering function.
2) CSSD Service Models & Centralized Governance
Common service models include:
- Hospital self-built CSSD (in-house reprocessing)
- Hospital provides services to nearby clinics/hospitals
- Government-supported regional CSSD
- Independent Medical Sterile Supply Center (MSSC)
- Public-private co-built regional service
Regardless of model, governance must cover structure management, process control, monitoring standards, and continuous improvement.
3) Facility Layout: Zoning & One-Way Flow (Dirty → Clean → Sterile)
- Scientific and rational layout
- Clear functional zoning
- Item flow from contaminated to clean areas (no reverse)
- Efficient workflow that reduces cross-contamination opportunities
4) Environmental Requirements: Pressure, ACH, Temperature, Humidity & Lighting
Ventilation & pressure zoning (core requirement):
- Mechanical ventilation is required.
- Decontamination areas should maintain relative negative pressure.
- Inspection/packaging/sterilization areas should maintain relative positive pressure.
| Area | Temperature (°C) | Relative Humidity (%) | Air Changes per Hour (ACH) |
|---|---|---|---|
| Decontamination | 16–21 | 30–60 | ≥10 |
| Inspection / Packaging / Sterilization | 20–23 | 30–60 | ≥10 |
| Sterile Storage | <24 | <70 | 4–10 |
Lighting guidance (task-based):
| Work Surface / Function | Typical Average Illuminance (lx) |
|---|---|
| General inspection | ~750 |
| Fine inspection | ~1500 |
| Washing sink area | ~750 |
| General work area / Sterile storage | ~300 |
5) The 10-Step Workflow & Risk Points
Standard CSSD workflow (10 steps):
Collection → Classification → Cleaning → Disinfection → Drying → Inspection & Maintenance → Packaging → Sterilization → Storage → Distribution
Infection-control risk points across the chain:
- Contamination stages clinical use, pre-treatment, collection, sorting, cleaning
- Potential contamination stages disinfection, drying, inspection & maintenance, packaging
- Sterilization safety stages sterilization, storage, distribution
6) Process Controls: Management Key Points by Step
6.1 Point-of-Use Pre-treatment (Clinical Area)
- Remove visible soil promptly; lumen devices must have internal soil removed.
- If instruments cannot reach CSSD within 1 hour, keep moist per IFU (including lumen).
- Heavily contaminated devices may be pre-soaked with enzymatic detergent to soften soil before cleaning.
6.2 Collection & Transport (Closed / Sealed Method)
- Prevent contamination of clinical environment.
- Avoid occupational exposure.
- Avoid repeated loading/unloading and device damage.
- Transport tools must be cleaned, disinfected, dried, and stored ready for use.
6.3 Cleaning (Prerequisite for Disinfection & Sterilization)
- Manual cleaning: suited for delicate/complex instruments and initial treatment of heavy organic soil.
- Mechanical cleaning: use validated programs; correct loading; dedicated racks; protect sharp/precision devices; open joints; disassemble where applicable.
6.4 Disinfection (Must Not Be Skipped)
- After cleaning, instruments should be disinfected—moist heat disinfection is preferred where applicable.
- Alternatives may include 75% ethanol, acidic electrolyzed water, or other disinfectants depending on device compatibility.
- Do not skip disinfection to save time after manual or mechanical cleaning.
6.5 Special Infection Scenarios: “Disinfect First, Then Clean”
- For prions, gas gangrene, or unknown outbreak pathogens: apply “disinfect first, then clean.”
- Follow WS/T 367 guidance: suspected/confirmed cases may prefer single-use devices; reusable contaminated items require double sealed packaging and dedicated CSSD handling.
6.6 Drying (No Natural Drying)
- Use drying equipment first; select temperature according to device material.
- For non-heat-resistant items: use low-lint cloth, pressure air gun, or ≥95% ethanol where appropriate.
- Lumen residual water must be removed (e.g., pressure air gun).
- Natural drying is not permitted.
6.7 Inspection & Maintenance
- Cleanliness: surfaces and joints free from blood/soil/scale; no rust.
- Function: joints move correctly; alignment and sharpness intact; no burrs/cracks.
- Use appropriate lubricants; do not use non-water-soluble products such as paraffin oil.
6.8 Packaging & Labeling (Sterile Barrier Integrity)
- Separate packaging for instruments and textiles.
- Correct assembly/placement; protect delicate and sharp items.
- Packaging material should comply with GB/T 19633 and relevant YY/T 0698 series categories.
- Labeling must be traceable (item name, packer ID, sterilizer ID, batch, sterilization date, expiry date, etc.).
6.9 Sterilization Method Selection & Key Restrictions
- Steam sterilization for heat/moisture-resistant items (preferred).
- Low-temperature methods for sensitive devices (EO, H₂O₂ plasma, low-temp formaldehyde steam).
- Chemical immersion is not used for surgical instrument sterilization.
- Rapid/flash sterilization is for immediate-use situations only and must not become routine.
- Lumen instruments should not be sterilized by gravity displacement steam sterilization.
- Follow manufacturer IFU for cycles and operation.
6.10 Storage & Distribution
- Store sterile items by category on racks in sterile storage areas.
- Rack spacing: ≥20 cm above floor, ≥5 cm from wall, ≥50 cm from ceiling.
- Follow FIFO; confirm validity, package integrity, and label clarity.
- Distribution records should include date, item, quantity, department, and sterilization date.
- Implants are released only after biological monitoring is qualified; emergency release requires monitoring logic consistent with guidance.
7) Quality Monitoring: Cleaning / Disinfection / Sterilization / B-D
7.1 Equipment Preventive Maintenance & Verification
- Follow manufacturer guidance for preventive maintenance and daily checks (washer-disinfectors, sealers, sterilizers).
- Steam sterilizers: annual parameter testing (temperature/pressure/time) and safety components (gauges/valves).
- Other sterilizers and sealers: annual verification per IFU.
7.2 Cleaning & Disinfection Quality Monitoring
- Daily: visual inspection and/or illuminated magnifier.
- Monthly sampling: randomly inspect all items inside 3–5 packs pending sterilization; record results.
- Washer-disinfector: per batch physical parameter monitoring; annually (or after program/load changes) use test soils for cleaning efficacy verification.
- Disinfection effect monitoring: items used directly after disinfection should be monitored quarterly per GB 15982 guidance (3–5 representative items per test).
7.3 Sterilization Effect Monitoring & Release Rules
- Physical monitoring failure: items must not be released; investigate and improve.
- External chemical indicator failure: items must not be released.
- Internal chemical indicator failure / wet packs: items must not be used.
- Biological monitoring failure: recall all unused items since last qualified BI; reprocess; investigate root cause; resume only after improvement and three consecutive qualified BI results.
- Implants: biological monitoring is required every batch; release only after BI qualification.
7.4 B-D Test (Pre-vacuum Steam Sterilizers)
- Perform daily empty-chamber B-D test before routine operation; use sterilizer only after a qualified result.
- After installation/relocation/major repair, repeat B-D test three times; use only after consecutive qualified results.
8) Traceability & Record Retention
- Maintain process records for cleaning, disinfection, sterilization, and equipment run parameters.
- For each sterilizer cycle: date, sterilizer ID, batch number, major load items, program, key parameters, operator ID, monitoring results.
- Retention: cleaning/disinfection records ≥ 6 months; sterilization monitoring records ≥ 3 years.
- Each sterile pack must be labeled with traceable information (item name, packer ID, sterilizer ID, batch, dates).
- Users should verify internal chemical indicator qualification, dryness, and cleanliness before use; information systems should support tracking back to CSSD.
9) Continuous Improvement & Recall Governance
- Establish continuous quality improvement measures and ensure timely problem handling.
- Establish sterile item recall system compliant with WS 310.3-2016 guidance.
- Regularly summarize and analyze monitoring data for sustained improvement.
10) External Instruments & Implants: Governance Requirements
10.1 Policy & Responsibility Boundaries
- Define responsibilities of functional departments, clinical departments, OR, and CSSD for external instruments/implants—handover, cleaning, disinfection, sterilization, and early release governance.
- Sign supplier agreements requiring full IFU (cleaning/disinfection/packaging/sterilization methods and parameters).
- Ensure sufficient processing time: elective cases delivered by a defined cutoff time; emergency cases delivered promptly.
- Establish dedicated posts and relatively fixed personnel for external instrument management.
10.2 Receiving Requirements
- Confirm supplier is hospital-approved.
- Maintain supplier and surgeon contact information.
- Collect and archive IFU documentation.
- Follow IFU for first-receipt testing and wet pack checks; archive results in information systems.
- For routine receiving: verify lists with supplier; both parties sign and archive records.
10.3 Post-use Handling
- Used external instruments must be cleaned and disinfected by CSSD before returning to suppliers.
11) Common Failures Seen in Audits (Real-World Risks)
- Skipping disinfection to save time after cleaning (manual or mechanical), creating a hidden safety gap.
- “Looks clean” without verification: missing routine visual/magnifier checks or monthly pack sampling records.
- Unstable pressure zoning: negative/positive pressure design exists, but daily operations (traffic/door opening) reverse airflow direction.
- Natural drying used as a shortcut, especially for lumen devices, increasing wet pack risk and sterilization failure.
- Packaging nonconformance: overweight packs, incorrect sizes for sterilizer type, or missing traceable labeling.
- BI failure handled incorrectly: no comprehensive recall since last qualified BI; no root-cause analysis; no three consecutive qualified BI verification after correction.
- External instruments governance gaps: missing IFU, unclear responsibility boundaries, elective delivery too late, or no archiveable receiving records.
12) Manager’s Readiness Checklist (Quick Audit)
Facility & Environment
- [ ] Functional zoning is clear; one-way item flow implemented.
- [ ] Negative/positive pressure strategy is verified and stable in daily operation.
- [ ] Temperature/RH/ACH targets are monitored and recorded.
- [ ] Lighting supports inspection tasks (general vs fine inspection).
Process Discipline
- [ ] Point-of-use pre-treatment rules applied (timeliness, moisture control, lumen care).
- [ ] Disinfection is not skipped after cleaning.
- [ ] Drying is equipment-based; natural drying is not used.
Packaging & Labeling
- [ ] Packaging materials comply with sterile barrier requirements; pack size/weight limits followed.
- [ ] Traceable labeling is complete and readable on every pack.
Monitoring & Release Control
- [ ] Physical/chemical/biological monitoring is implemented with clear “no release” rules.
- [ ] Daily B-D test is performed for pre-vac steam sterilizers.
- [ ] BI failure triggers recall, corrective action, and three consecutive qualified BI verification.
- [ ] Implant batches follow BI requirements for every batch before release.
External Instruments
- [ ] Supplier approval + IFU archive exists and is accessible.
- [ ] Receiving records are signed and stored; post-use reprocessing is enforced before return.
13) FAQ
What is the difference between disinfection and sterilization in CSSD?
Disinfection reduces microbial load and protects staff/environment, while sterilization eliminates all microorganisms including spores. Disinfected items are not sterile and must not be treated as such.
Why is cleaning considered the most critical step?
Cleaning removes organic soil that can shield microorganisms. Many sterilization failures originate from inadequate cleaning rather than sterilizer malfunction.
Can disinfection be skipped after mechanical cleaning?
No. Disinfection must not be skipped after manual or mechanical cleaning to save time.
What happens if biological monitoring fails?
Recall all unused items since the last qualified biological monitoring result, reprocess them, analyze root causes, implement correction, and resume only after three consecutive qualified biological monitoring results.
How long should CSSD records be retained?
Cleaning/disinfection records should be retained for at least 6 months, while sterilization monitoring records should be retained for at least 3 years.
14) Recommended Internal & External References
Suggested ICARELIFE Internal Links (replace placeholders)
- Hermetic Doors for Hospital Environments — pressure zoning & airtight separation
- Duct-Free Laminar Ceiling Explained — airflow protection strategy for OR
- Choosing an AHU for Operating Theaters — operational stability & control strategy
- Medical Airflow Systems Overview — HVAC zoning and airflow organization
- Operating Room Wall Panel Systems — architectural support for clean zoning
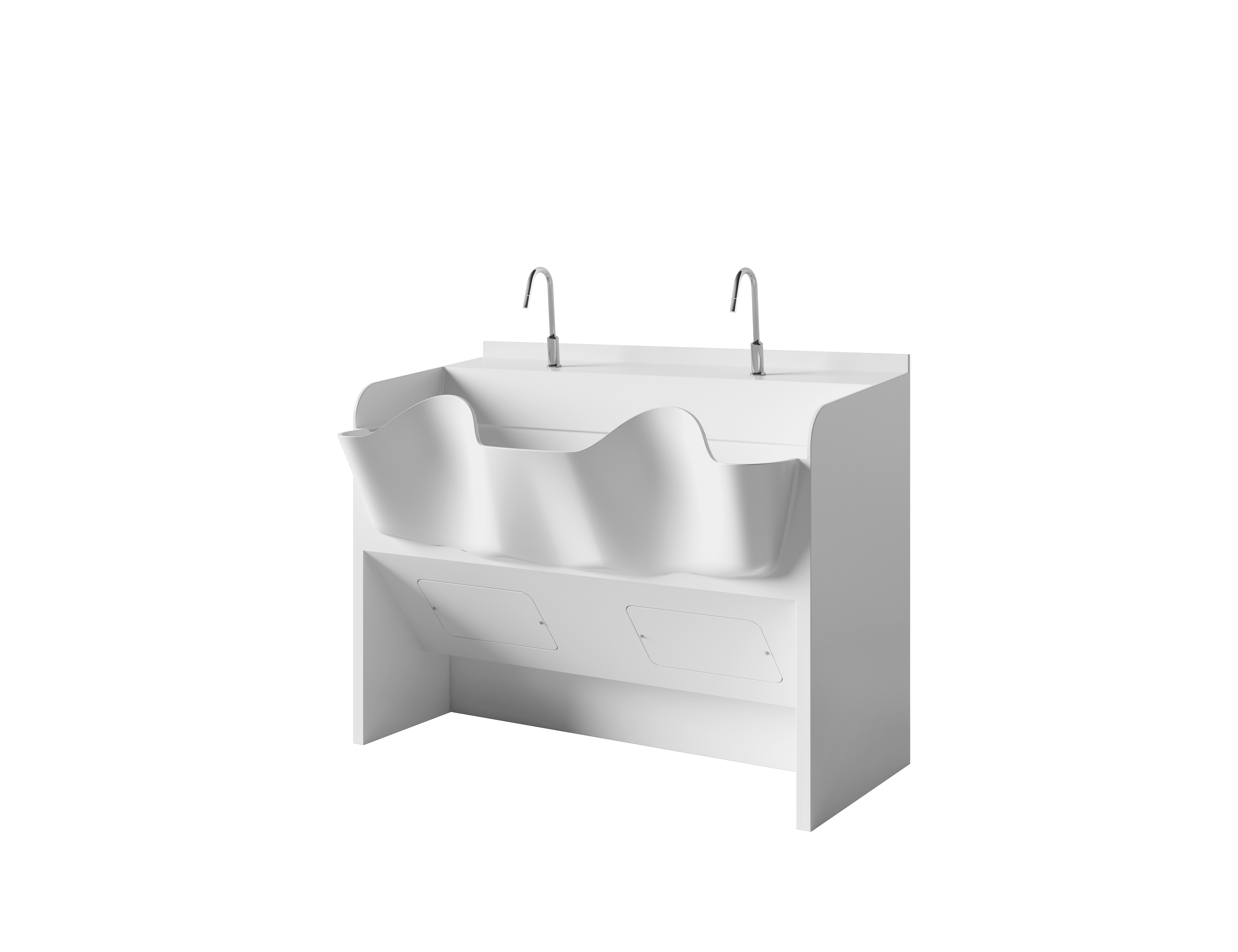
- Corian® Solid Surface Surgical Scrub Sink — workflow support for hand hygiene
External references (as cited in the PPT)
- WS/T 367 — management of special infection device reprocessing (as referenced)
- WS 310.3-2016 — sterile item recall governance requirements (as referenced)
- GB/T 19633 — sterile barrier system packaging materials (as referenced)
- YY/T 0698 series — packaging material categories (as referenced)
- GB 15982 — disinfection effect monitoring reference (as referenced)
Source basis: CSSD infection-control management key points (training material). This guide translates requirements into management and governance language for education and infrastructure planning.